
Data Integrity & Compliance Features
Safe. Powerful. Compliant.
In all regulated industries, especially pharmaceuticals and biotech, regulatory compliance of electronic data is crucial. Seamless data governance with full data integrity and data security are a must.
With advanced yet simple-to-use features, Anton Paar software solutions uphold the highest industry standards as per FDA 21 CFR Part 11, EU Annex 11, PIC/PICS 041-1, and GxP – with a focus on GMP and cGMP.
Meticulous lab instrument qualification and system validation ensure that Anton Paar instruments and systems strictly follow USP <1058> principles and a risk-based lifecycle approach. Their comprehensive and streamlined software features simplify data transfer, review, and storage – reinforcing the highest data integrity standards at any point of action.
Prioritizing preventive actions mitigates non-conformity risks, yet saves time and resources, and ensures audit readiness at any time.
The power of reliable data integrity and compliance excellence
Our comprehensive documentation services ensure that our clients receive compliant, system-specific documentation for initial qualification and validation, maintenance, and requalification. We understand the critical importance of maintaining data integrity and offer a wide range of resources and support to meet the unique needs of each category listed below.
| Data Integrity Categories All 21 CFR Part 11 compliant |
||||
| Excellence | Advanced | Standard | ||
| Audit Trail | ||||
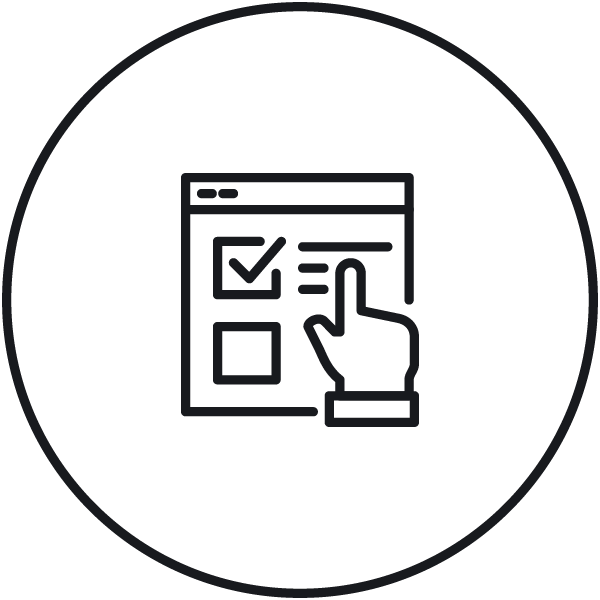 |
Audit trail mode Secure electronic records of system activities and actions (measurements, signing, exports, setting changes) with comment option. |
|||
| Electronic Signature | ||||
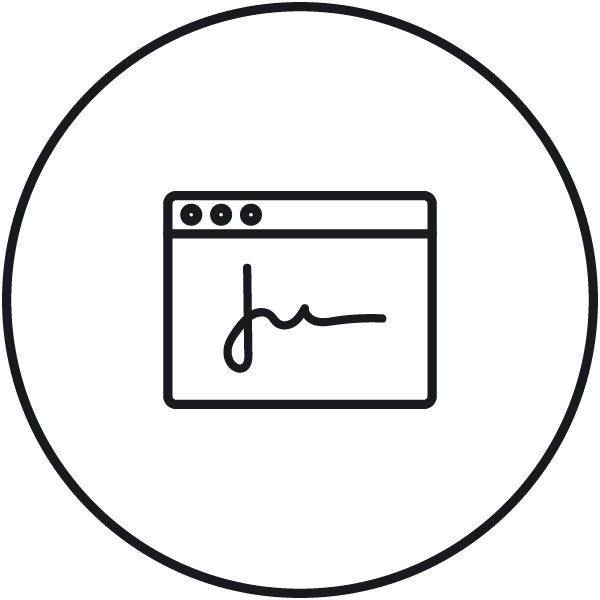 |
Electronic signature Electronic verification of electronic data on different signing levels with comment option. |
|||
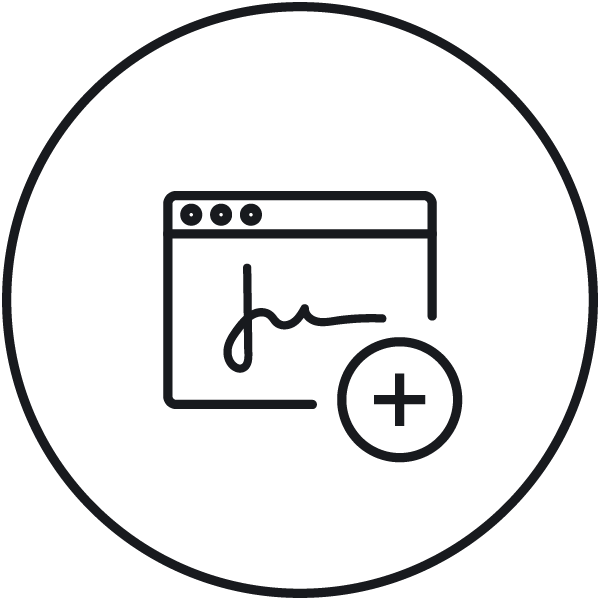 |
Enhanced electronic signature Enhanced e-signature options for one-step/two-step signing (submission/approval only) and substitute signing. |
|||
| Data Security | ||||
 |
Data printout Fully traceable, GxP-compliant printout of data and instrument settings. |
|||
 |
Protected data storage "Ring-buffer" memory deactivation with stop of measurement operation prior to data removal. |
|||
 |
Restricted time and date setting | |||
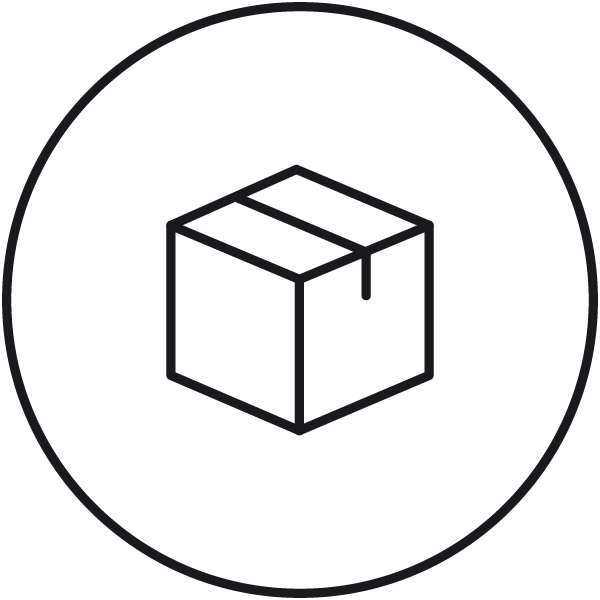 |
Data backup Data backup (measurements, checks, calibrations, settings, etc.), including instrument-specific disaster recovery. |
|||
 |
Electronic data transfer Data transfer using standard protocols (LAN, network share) or Anton Paar lab execution software, AP Connect Pharma. |
|||
| User Management | ||||
 |
User management with fixed password rules Creation and identification of users on three predefined levels (operator, manager, and administrator). |
|||
 |
Individual user group management Creation of user groups for individual user group management (e.g., start/stop, checks, change of settings, etc.). |
|||
 |
Configurable password security Enhanced security using complex passwords (upper/lower case, number, special character), and defined password expiration. |
|||
| Enhanced Compliance Features | ||||
 |
Configurable visibility of measurement values No real-time data on instrument display during measurement. Only final results are shown to avoid any user influence. |
|||
 |
Raw data access and storage Possibility to store, access, and review raw data after measurement. For some systems via dedicated software viewer. |
|||
 |
Guided, selectable check limits & validity Configurable limits to verify the validity of measurement data in regular (optional operationally mandatory) instrument checks. |
|||
 |
Non-storage mode (with audit trail) No data except for audit trail stored on the instrument. Immediate printout after each measurement necessary. |
|||
 |
Non-deletable data mode Function "archive" replaces function "delete" for secure data export and subsequent data removal. |
|||
 |
External system control Desktop software for system operation and control. For some instruments optional to embedded operation. |
|||
| Compatible Products | Show all Excellence instruments |
Show all Advanced instruments |
Show all Standard instruments |
|

Data Integrity, Security and Compliance in Regulated Industries
Understand 21 CFR Part 11 requirements. Delve into detailed descriptions of individual features. Explore how measuring technology and pharmacopeia chapters relate.
Download the comprehensive e-book “Data Integrity, Security and Compliance in Regulated Industries.”
Contact us for more information.