Pharmaceutical compliance in the fields of viscometry and rheometry
MASTER THE FLOW and be compliant
Anton Paar’s viscometers and rheometers are the perfect instruments for characterizing and improving your materials’ structure and consistency. You can be sure that all instruments provide the following:
- Full compliance with all pharmaceutical regulations of 21 CFR Part 11 and full data integrity*
- Features that make it easy to measure challenging samples of any type and help avoid user errors
- A high level of modularity regarding accessories and measuring techniques
- A unique pharma qualification service that helps you save an enormous amount of time and money during instrument qualification
- A global application and service network, broad application knowledge, and local support in the regional technical centers
* ViscoQC 100 provides limited features for pharmaceutical compliance
MASTER THE FLOW and find your solution
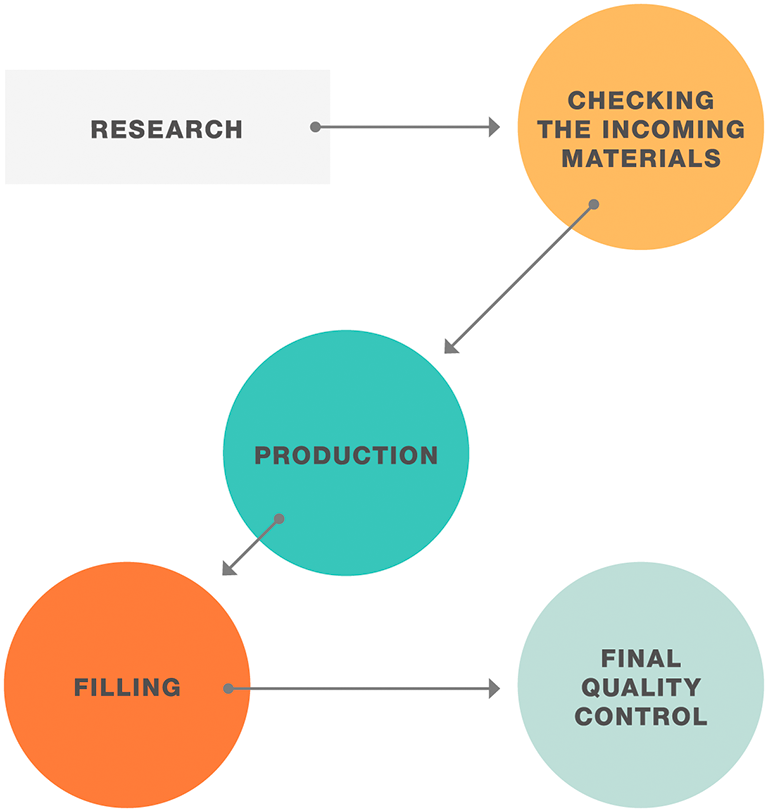
Everything flows, also when it comes to pharmaceutical manufacturing, quality control, and research. Anton Paar offers a variety of solutions for determining the flow and deformation behavior of a variety of materials – from oils used as raw materials to end products such as cough syrup, gels, evaporation fluids, and even catheter polymers and pills in powder form.
| Challenge | Solution | Your benefit | Instrument |
The moisturizing effect of the eye drops only lasts for a few seconds. | Analyze and precisely quantify the viscosity values of the methylcellulose solution in the eye drops at different temperatures, simulating real-life conditions (shear thinning effects during eye blinking and body temperature). | You can simulate the consistency of the eye drops by determining the viscosity values at different shear rates and temperatures. Knowing these values you can then adapt the portion of methylcellulose solution within the eye drops. | |
After approx. 6 months of storage the skin gel hardens in the tube. | Check the storage stability with a frequency sweep and thus observe and avoid separation of the two phases within the gel. | Fast and precise frequency sweeps can be performed with rheological oscillation tests. Predefined test templates enable you to characterize your gel within a few minutes with varying xanthan content. | |
The cough syrup does not stay on the spoon and flows through the digestive tract too fast (so the surface of the throat is not covered). | Determine single-point viscosity for quick quality checks or make multi-point analyses at a constant temperature with an increasing speed. | You can ensure the soothing effect and right viscosity of the cough syrup by using a single-point viscosity check or by studying the flow- or temperature-dependent behavior. For a more in-depth analysis of the flow behavior of the cough syrup, the mathematical model „shear thinning index“ can be used. | |
It is not possible to pump and squeeze ointments out of the packaging. During application on target body areas, good spreadability is not given. | Determine single-point viscosity for quick quality checks or perform multi-point analyses at a constant temperature with increasing speed/shear rate. | You can ensure perfect pumpability and spreadability of ointments by using a single-point viscosity check or by studying the flow behavior and the yield point. | |
The microemulsion separates into phases in heat (35 °C). | Analyze the temperature-dependent flow behavior at a constant speed/shear rate. | You can simulate the product’s behavior during storage at different temperatures and improve the formula accordingly. | |
After squeezing the ointment out of the tube the sample remains too thin. | Analyze the structural regeneration with a step test: 3 interval thixotropy test (3ITT). | With such a test you can simulate the real sample behavior during application. Interval 1 simulates the rest phase before processing. Interval 2 serves as a simulation of shearing during the application. Interval 3 describes the rest phase and structural regeneration after application (squeezing out of the tube/filling/pumping). | |
Clinical nourishments cannot be swallowed easily. | Determine single-point viscosity for quick quality checks or perform multi-point analyses at a constant temperature with increasing speed/shear rate. | By analyzing the viscosity at low and high shear rates the viscosity of clinical nourishments at rest and during swallowing can be determined. Adding thickening agents to a low-viscosity fluid makes the fluid more viscous, which makes swallowing easier. | |
The pills disintegrate during the production process or storage. | Determine the cohesion strength of the powder which is used to press the pills. | You can figure out the critical parameters for the cohesion strength of your raw material and minimize the reject rate in the production process by using only powders with suitable properties and adjust the production parameters in a target-oriented way. | |
The particulate raw material tends to segregate or demix during the production process. | Check the segregation and wall friction behavior of your granulate matter. | You can avoid negative influences on the effectiveness of the active pharmaceutical and problems in the production process due to changed powder flow behavior by adjusting the formulation of the raw material. | |
The artificial joints show signs of wear very early after implantation. | Check the tribological behavior (friction, wear, lubrication) of the material combination used for the artificial joints. | You can investigate and optimize suitable material combinations and surface treatments to ensure the required long endurance of the artificial joints within a human body. | |
The hand sanitizer (liquid and gel) just doesn’t feel right when applied on the hands. | Analyze the pumpability and spreadability with rotational- and how the product feels on the skin with oscillatory rheological measurements. | You can optimize the users’ experience by getting the rheological properties right, so the gel is easily dispensed, spreads nicely, and at the same time isn’t too liquid. | |
Upscaling production of vaccinations is difficult due to mixing problems of the components. | Find out the ideal processing and mixing parameters by analyzing rheological properties. | You can design the upscale from small lab-scale to large industrial processes by knowing the viscous properties. And you can also precisely control the quality during manufacturing. | |
When using methylcellulose the sample viscosity seems to change during production. | Measure the viscosity in dependence of an applied shear rate. | You can find the right processing parameters for each solution and concentration for maximum throughput without loss of material. | |
Applying dental filling composites in a patients’ tooth is difficult due to their shear thickening behavior during the filling process. | Analyze the cross-linking process and setting of composites. | You can guarantee unchanging behavior for easy use and application of dental filling composites. | |
The environmental humidity renders the powder processing (e.g. for tableting) in the factory difficult. | Measure the impact of humidity on powder flow and other powder characteristics. | You can optimize the environment and processing parameters and thereby avoid expensive down-time during manufacturing. |
You did not find your specific sample? Anton Paar still has the solution for your challenge. Just contact us for more information.

The whole world of viscometry and rheometry
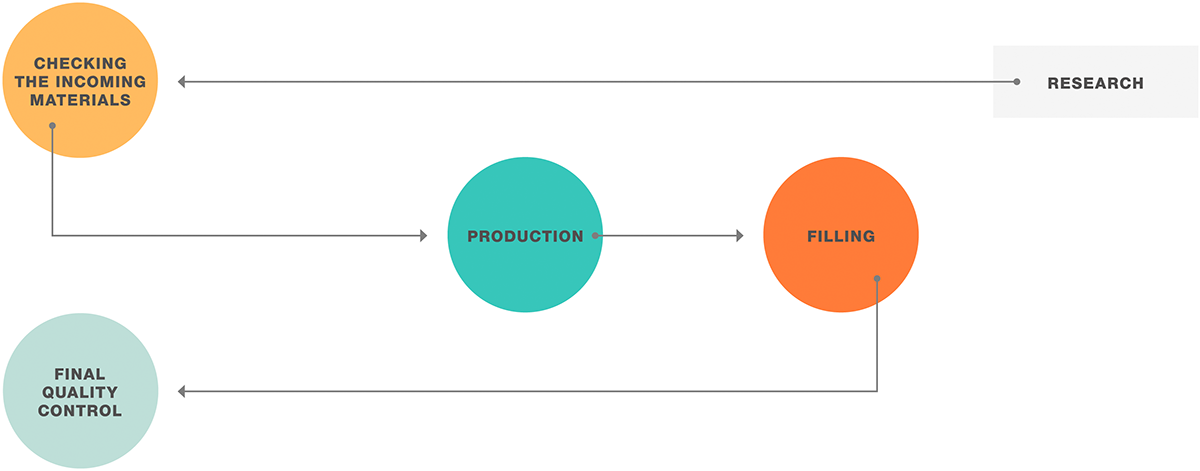
Flow and deformation behavior are essential parameters when it comes to material characterization. Viscometers and rheometers are the ideal tools to see whether your sample is in the right flow.
MASTER THE FLOW with pharmaceutical guidelines
Fulfilling necessary regulations can be challenging for every pharmaceutical company. Anton Paar supports you with the fulfillment of Good Manufacturing Process (GMP) and pharmacopeia standards. You will benefit from Anton Paar’s comprehensive state-of-the-art portfolio of rotational viscometers and rotational/oscillatory rheometers at every step of the process.
rheometers
rheometers

3-year warranty
- Effective January 1, 2020, all new Anton Paar instruments* include repair for 3 years.
- Customers avoid unforeseen costs and can always rely on their instrument.
- Alongside the warranty there is a wide range of additional services and maintenance options available.
* Due to the technology they use, some instruments require maintenance according to a maintenance schedule. Complying with the maintenance schedule is a prerequisite for the 3-year warranty.
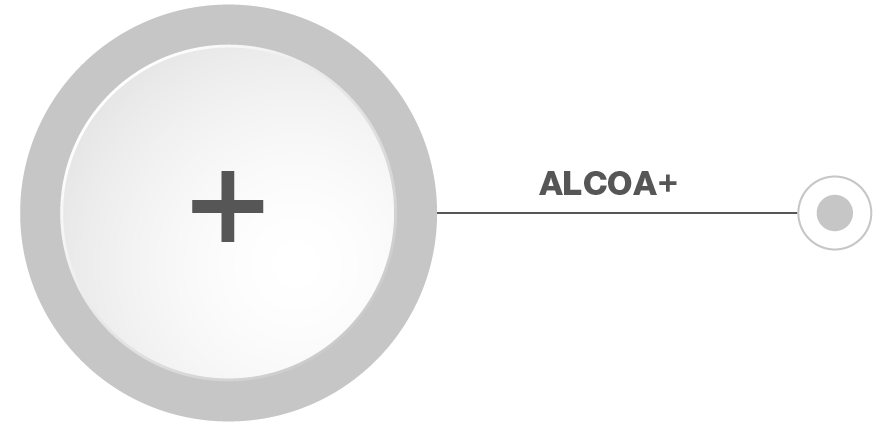
ALCOA+
The path to full data integrity is ALCOA+ and its fundamental principles of data quality. Data integrity is key in the pharmaceutical industry and is becoming increasingly important for GMP and compliance in this industry in general.
ALCOA+ means that all data – printed or electronic – has to be:

Attributable: Data must be recorded accurately and it has to be clear who performed the action and when.
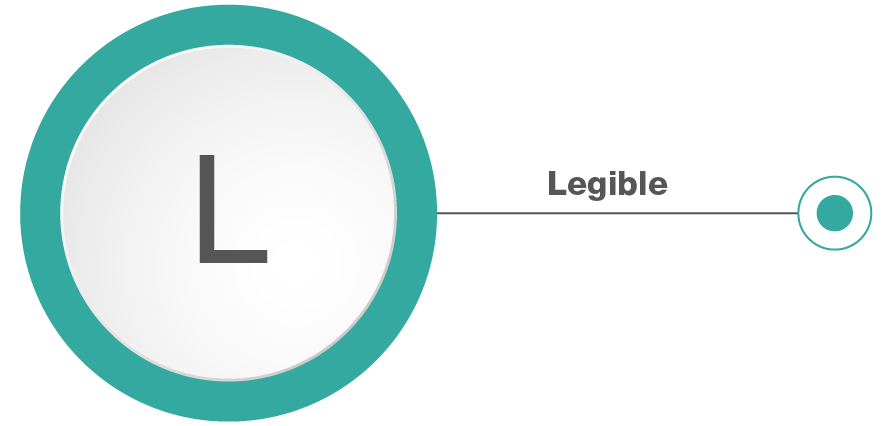
Legible: Data must be permanently recorded across the entire life cycle in a durable medium and has to be readable.
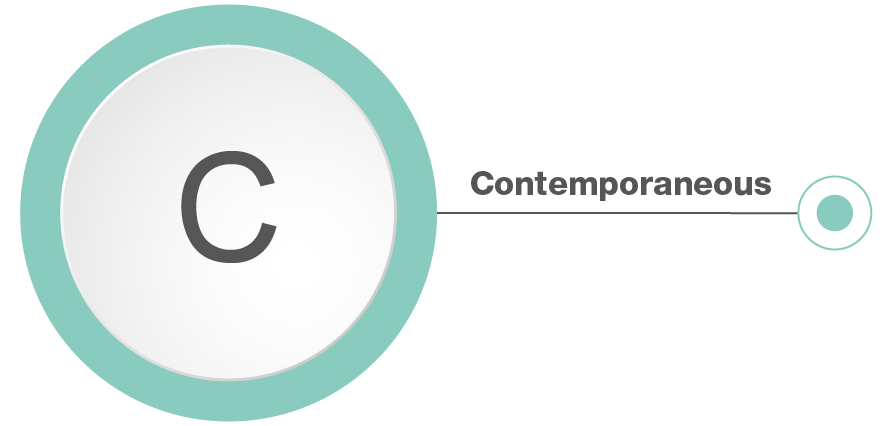
Contemporaneous: Data must be recorded at the time work is performed.

Original: Data must be an original record or certified true copy.

Accurate: Data must not contain errors or performed edits without documented changes (full audit trail); data must be reliable.
Complete: Data must include repeated tests or re-analyses performed on the sample.
Consistent: Data must be provided with date and time stamps of the analysis in the expected sequence and in chronological order.
Enduring: Data must be documented on worksheets, laboratory notebooks, or electronic media.
Available: Data must be accessible for review/audit for the lifetime of the record.
Anton Paar has the solution for your challenge. Contact us for more information.

Master the flow of your pharmaceuticals
Do you have the feeling that tedious yearly audits by FDA or any other regulatory are a hurdle and put a lot of stress on you and your colleagues? Let Anton Paar bring you to smooth audits of your rotational viscometers and rotational/oscillatory rheometers and lean back.
Watch nowApplications

Software Features of Anton Paar Instruments for the Pharma Industry