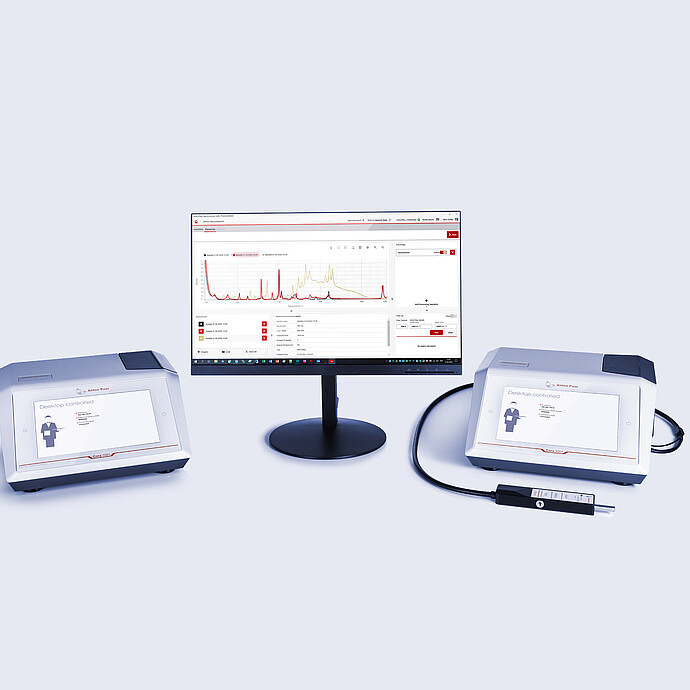
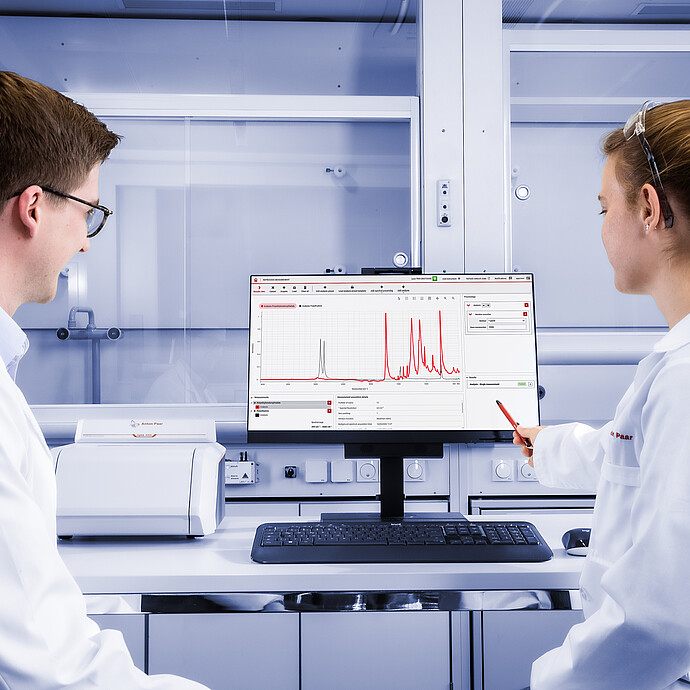
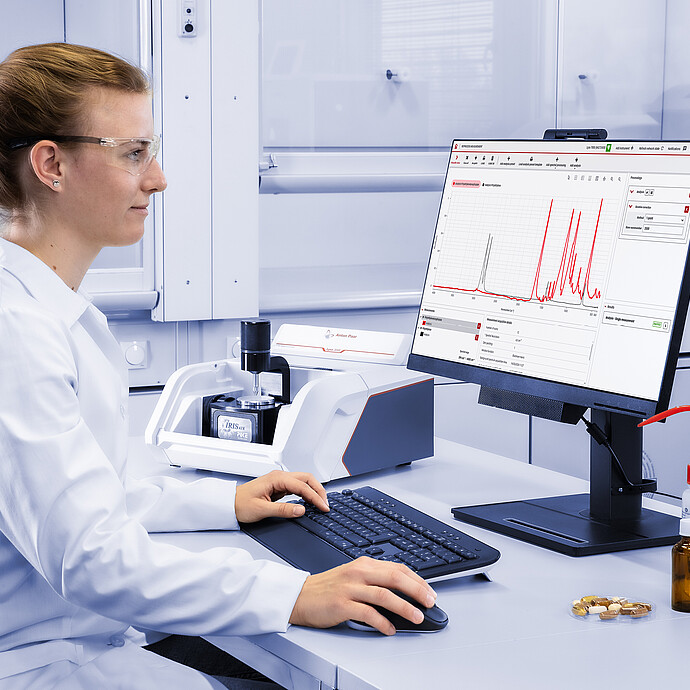
Spectral Analysis Software:
AP Spectroscopy Suite
- Control of Cora 5001 (Raman) and Lyza 7000/3000 (FTIR) from a PC
- Chemical identification of samples within seconds
- Convenient method development and spectral processing
- Lifetime data integrity and audit trail with search function
- Transparent and secure electronic signing and versioning
- Pass pharma audits with lifetime data integrity and audit trail
- Achieve trusted results through electronic signatures
- Perform complex spectroscopy tasks with ease
- Operate FTIR and Raman instruments from one PC
- Link approved results with other systems using AP Connect
Perform chemical identification and verification of substances with Cora 5001 and Lyza 7000/3000 in seconds, with Anton Paar’s spectral analysis software: Spectroscopy Suite.
The ideal solution for incoming goods inspection, quality control of final products, and R&D in both regulated and non-regulated environments, Spectroscopy Suite takes all the complicated spectroscopy aspects out of daily measuring routines. Predefined workflows and settings – designed to adhere to stringent internal and external compliance regulations – speed up analysis and prevent operator error. Methods, reference data, and libraries are thoroughly versioned and can be configured for review and approval using electronic signatures.
Key features
Combined spectroscopy power
Both the FTIR and Raman molecular spectroscopy methods can be controlled by the same spectral analysis software. You can use AP Spectroscopy Suite individually as Raman analysis software or FTIR analysis software, or combine for a powerful analytical workstation. Specialists only need to operate one system, making workflows more efficient. Specialists and operators alike enjoy ease-of-use as they complete their tasks.

Increase confidence in your identification results
Individual configuration ensures only approved methods can be followed, making the day-to-day running of samples safe and efficient. Guided workflows optimize conditions and eliminate human influence on results. Our spectral analysis software automatically compares the measured spectra with your own inhouse libraries, the carefully maintained Anton Paar substance libraries, and optional third-party libraries. Identification results appear in a clear pass/fail answer. A valid system suitability test is a prerequisite for starting a measurement and ensuring correct results. The strict versioning concept for methods and libraries increases transparency and simplifies management of the latest valid workflows.

Simplify your daily routine with predefined methods
All sample-specific measuring settings and reference data are defined by configurable methods, which make it easy for everyday operators to achieve high quality results – even for complex spectroscopy tasks. Methods can only be created or changed by user groups with the corresponding privileges. Each method is automatically versioned and can also be configured to undergo a guided review and approval process using electronic signatures, ensuring that only approved methods can be used in the AP Spectroscopy Suite for analysis.

Easy reference library generation
Just like the methods, reference datasets can only be generated by users with the rights to do so. The process is guided and straightforward. Displaying multiple spectra assists in selecting the right reference data sets, and the reference libraries are automatically versioned. Again, the configurable review and approval processes ensure that only approved substances can enter a library, and that only approved libraries can be used for analysis with the spectral analysis software. Anton Paar’s Spectroscopy Suite also allows you to turn off all approvals, e.g. for quick tasks in R&D and quality control in unregulated areas.

The best choice for spectroscopy in regulated environments
With AP Spectroscopy Suite Premium, all actions and data are traceable and searchable, helping you pass audits in regulated environments. Functionalities like access control via active directory, electronic signing, user group administration, audit trail, data management, export, backup, and restore ensure compliance with 21 CFR Part 11, GAMP 5, and EU GMP Vol. 4 Annex 11. Anton Paar’s Analytical Instrument and System Qualification Package (AISQ+) minimizes the effort and time needed to prepare for go-live.

Standards
CFR
GAMP
EU GMP
European Pharmacopoeia (Ph. Eur.)
US Pharmacopoeia (USP)
Japanese Pharmacopoeia (JP)
Chinese Pharmacopoeia (ChP)
IP
Standards
CFR
GAMP
EU GMP
European Pharmacopoeia (Ph. Eur.)
US Pharmacopoeia (USP)
Japanese Pharmacopoeia (JP)
Chinese Pharmacopoeia (ChP)
IP
Anton Paar Certified Service
- More than 350 manufacturer-certified technical experts worldwide
- Qualified support in your local language
- Protection for your investment throughout its lifecycle
- 3-year warranty
Compatible instruments

Cora 5001 Direct

Cora 5001 Direct Pharma

Cora 5001 Direct Standard
Cora 5001 Fiber

Cora 5001 Fiber Pharma

Cora 5001 Fiber Process Monitoring

Cora 5001 Fiber Standard

Lyza

Lyza 3000

Lyza 7000