Pharmaceutical Powder Characterization
Flowability, solubility, consistency, purity, and stability are parameters to be considered when it comes to optimizing pharmaceutical powders. A wide range of analytical instrumentation focused on pharmaceutical powder characterization under authentic conditions will help you hone the efficacy of the product.
Deepen your understanding of particle properties to get more predictable formulations. Understand the flow behavior of your powders at every step – from formulation to production. Prevent unconsidered environmental conditions from affecting production efficiency and quality.
Obtain reliable and repeatable results for parameters such as surface area, particle size and distribution, bulk and tapped density, powder flow properties, and more. Gather insights on polymorph properties at non-ambient temperatures via X-ray diffraction. Identify active pharmaceutical ingredients and excipients with Raman spectroscopy. Prepare your samples for inductively coupled plasma mass spectrometry via closed vessel digestion.
Download the e-book, “The Field Guide to Pharmaceutical Powder Characterization,” and find out more.

The Must-Read Anton Paar E-Book: “The Field Guide to Pharmaceutical Powder Characterization”
Deepen your understanding of materials and exercise control over pharmaceutical powders. This e-book, focused on pharmaceutical powder characterization and containing real measurement data, tells you how to deal with pharmaceutical powder challenges such as flowability, solubility, and consistency under real-life conditions.
Solubility, Dissolution Rates, and Consistency

Optimizing drug formulation consistency, dissolution rates, bioavailability, and eventual absorption behavior is critical during drug development, testing, and administration. One of the primary physical proprieties that directly affects each of these parameters is the particle size of the formulation. Quickly and accurately measuring the particle size and size distribution of a drug formulation and how it changes over time and under different conditions is often the first step in developing a novel formulation.
Additionally, the surface area and porosity of a powder pharmaceutical formulation provide even more insight, particularly into solubility and dissolution rates. Specifically: the greater the overall surface area and the more pores present in a powder, the better the solubility and dissolution rate. Accurately measuring the particle size as well as the surface area and porosity of pharmaceutical powders is critical for optimizing drug consistency and delivery. It’s also important for controlling the release rate of the drug in the patient.
Powder Flow

The processing, handling, and storing of powders on an industrial scale causes issues that are typically not seen on a pilot scale. Many powders show a significant behavioral dependence on the environmental conditions they’re subjected to (e.g., temperature and humidity). These behavioral changes express themselves as changes in properties such as flowability, cohesion, tendency towards agglomeration – and many more. These usually unwanted effects can be problematic when, for example, excipient and active pharmaceutical ingredients separate or segregate. This change of flow properties can pose a challenge during processing and filling.
Determining temperature/humidity-dependent powder rheological properties enables the characterization of flow and mechanical behavior of pharmaceutical powders. Analyze your powder formulations and ensure you set the right preparation conditions. Understand flow properties with powder rheology for efficient and flawless transportation, processing, and mixing.
Packing and Tableting
Solid density is an important property of pharmaceutical powders – drugs or excipients. Bulk and tapped density are related to the flow properties of powder. The flowability of a powder, in particular of an additive or excipient, is important in determining how that additive will affect the granulation or tableting process. Good flowability ensures that there is appropriate, uniform filling of capsules so consistent weight and dosage is achieved.
Determining the open porosity of a tablet is critical to understanding many different tablet properties, including strength and shelf life. Porosity is also important in preventing excipient fracture during tablet compression.
Gas pycnometry measurements provide skeletal density data that let you assess powder flow information and tablet porosity.
Purity, Stability, and Percent Crystallinity (XRD and SAXS)

Long-term stability of pharmaceutical powders and how, for example, storage conditions and packaging affect the stability of such powders are of key interest. Methods such as X-ray diffraction (XRD) and small-angle X-ray scattering (SAXS) can be used to analyze powders for aging effects that alter the degree of crystallinity (the ratio between amorphous parts and crystalline phases) or create other structural changes. These will substantially change over time and with the storage conditions of the powders. SAXS and XRD are capable of measuring samples under ambient and non-ambient conditions (and in situ, where required) giving essential insights into aging, stability, appropriate packaging, and optimal storage conditions.
The crystal structure of any active pharmaceutical ingredient (API) determines the product stability, solubility, and, ultimately, bioavailability. XRD is the standard method of identifying crystalline phases and conducting polymorph screening of APIs to understand the forms present in a powder.
Sample Preparation for Elemental Purity Analysis

Since elemental impurities constitute not only a toxicological risk for the patient but may also affect the quality and efficacy of pharmaceutical products, analysis of them plays an important role in the development and quality control of pharmaceuticals.
The quantification of elemental impurities is performed by means of ICP-OES or ICP MS together with reliable sample preparation techniques like microwave-assisted acid digestion. The usage of this particular sample preparation method ensures excellent recovery rates, low variations, and high sample throughput.
Sample Identification
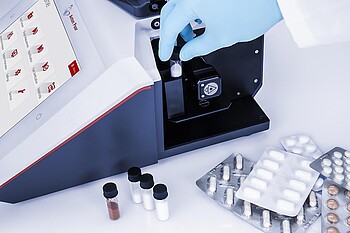
The pharmaceutical production industry is one of the most heavily regulated global markets. This guarantees product quality and minimizes risks for patients worldwide. Identity tests are required under international pharmacopeias and GMP guidelines for all APIs, excipients, drug products, and packaging materials. According to US Pharmacopeia chapters 197 and 858, identity tests may be conducted using Raman spectroscopy and must be validated according to USP 1225.
Fast and accurate methods are required to quickly assess whether a batch is fit for further use or needs to be rejected. Identity testing solutions have to comply with the FDA’s CFR 21 Part 11, and data integrity and a complete audit trail must be ensured.